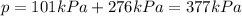Answer:
377 kPa
Step-by-step explanation:
The absolute pressure of a gas is given by the sum of its gauge pressure and the atmospheric pressure:
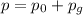
where
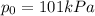 is the atmospheric pressure
is the atmospheric pressure
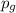 is the gauge pressure of the gas
is the gauge pressure of the gas
In this problem, the gauge pressure of the gas is
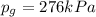 . Therefore, the absolute pressure is
. Therefore, the absolute pressure is