Answer:
Raising its temperature by
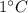
Step-by-step explanation:
The specific heat capacity of a substance is defined as the amount of energy needed to raise the temperature of 1 kg of a substance by
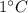 .
.
The specific heat capacity of a substance essentially tells us how much energy is needed to heat the substance: the larger it is, the more energy is needed. The amount of energy needed to increase the temperature of a substance is given by
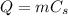
where
m is the mass of the substance
Cs is the specific heat capacity
 is the temperature variation of the substance
is the temperature variation of the substance