Answer : The complete equation will be,
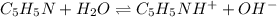
Explanation :
According to the Bronsted Lowry concept, Bronsted Lowry-acid is a substance that donates one or more hydrogen ion in a reaction and Bronsted Lowry-base is a substance that accepts one or more hydrogen ion in a reaction.
The given reaction will be,
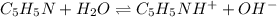
In this reaction,
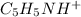 is act as a Bronsted Lowry-base by accepting one hydrogen ion from the water and
is act as a Bronsted Lowry-base by accepting one hydrogen ion from the water and
 is act as a Bronsted Lowry-acid by donating one hydrogen ion to pyridine.
is act as a Bronsted Lowry-acid by donating one hydrogen ion to pyridine.