Answer: Option (D) is the correct answer.
Step-by-step explanation:
It is known that density of water or 100 grams of water is 1 g/ml. As density is mass divided by volume then it is known that in 100 grams of water 99.2 grams of KBr can be dissolved at
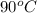 .
.
Therefore, it is obvious that solution will be unsaturated because 50 grams of KBr will completely dissolve in it.
As saturated solution is a solution which is unable to dissolve more solute. Whereas a solution is unsaturated when solute dissolves in it until the saturation point.
Hence, we can conclude that if Marie and Calvin dissolve 50 grams of KBr in 100 grams of water at 90oC, the solution is unsaturated.