Avagadros law states that at constant pressure and temperature conditions the volume is directly proportional to number of moles of gas
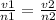
where v1 is volume and n1 is number of moles at first instance
v2 is volume and n2 is number of moles after some of the gas is released
substituting the values in the equation
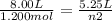
n2 = 0.788 mol
after some of the gas is released only 0.788 mol remain