Answer: The correct answer is Option A.
Step-by-step explanation:
Velocity of the gas is inversely related to the molar mass of the given gas. The equation representing the relation between the two is:
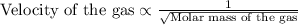
From the above relation, if the molar mass of the gas is more, the velocity of the gas will be less and vice-versa.
The molar masses of the given gases are as:
Ne = 20 g/mol
 = 32 g/mol
= 32 g/mol
Ar = 40 g/mol
 = 28 g/mol
= 28 g/mol
As, the molar mass of neon is the lowest. Thus, it will have the highest velocity.
Hence, the correct answer is Option A.