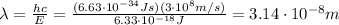Answer:
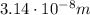
Step-by-step explanation:
The energy of a photon is related to its wavelength by

where
E is the energy
h is the Planck constant
c is the speed of light
 is the wavelength
is the wavelength
In this problem, we know the energy
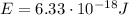
So we can solve the previous formula for the wavelength: