Answer: Option (a) is the correct answer.
Step-by-step explanation:
A covalent bond is defined as the bond which is formed due to sharing of electrons.
For example, a carbon atom has 4 valence electrons and a hydrogen atom has 1 valence electron.
So, when a carbon atom chemically combines with a hydrogen atom then sharing of electrons take place between the two and it results in the formation of a methane molecule.
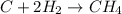
Whereas a bond in which transfer of electrons take place from one atom to another is known as an ionic bond.
For example,
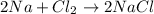
Thus, we can conclude that in a covalent bond electrons that are shared between atoms holds atoms together.