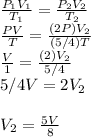Answer:
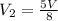
Step-by-step explanation:
I am assuming you are saying what is the final volume of the gas
Known :
Initial volume (V1) = V
Initial temperature (T1) = T
Final temperature (T2) = 5/4 T
Initial pressure (P1) = P
Final pressure (P2) = 2P
Wanted: Final volume (V2)