Answer: The correct answer is Option D.
Step-by-step explanation:
Endothermic reactions are defined as the reactions in which energy is absorbed by the system. The total enthalpy change of the reaction comes out to be positive.
Exothermic reactions are defined as the reactions in which energy is released by the system. The total enthalpy change of the reaction comes out to be negative.
Decomposition reaction is defined as the reaction in which a large substance breaks down into two or more smaller substances.
Photosynthesis is defined as the process by which the producers make glucose molecule with the help of carbon dioxide and water in the presence of sunlight.
The chemical equation representing this reaction follows:
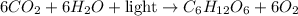
As, the energy term is written on the reactant side. This means, that the energy is getting absorbed by the system and is considered as an endothermic reaction.
Hence, the correct answer is Option D.