Answer:
2.0158 grams
Explantion:
We are to find the mass of the hydrogen atoms in 1 mole of water.
We know that the formula of water is:

We can see, from the above mentioned formula, that water has 2 hydrpgen atoms.
From the periodic table, we get to know that Hydrogen has an atomic mass of 1.00794 grams.
As there are 2 atoms of hydrogen in water so
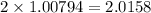 grams is the answer
grams is the answer