Answer: The correct answer is Option B.
Step-by-step explanation:
When aluminium metal reacts with air, it produces an oxide named as aluminium oxide. The chemical formula for this compound is
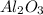
The balanced chemical equation for the above reaction follows:
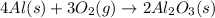
By Stoichiometry of the reaction:
4 moles of aluminium metal reacts with 3 moles of oxygen to produce 2 moles of aluminium oxide.
This reaction is a type of synthesis reaction because substances are reacting in their elemental state to produce one compound.
Hence, the correct answer is Option B.