Hello!
The answer is: 30 calories
Why?
From the statement we have:
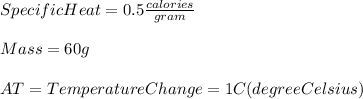
We can calculate heat transferred using the following equation;
q=mCΔT
Where:
q=HeatTransferred
m=mass=60g
C=SpecificHeat=0.5\frac{calories}{g}
ΔT=TemperatureChange=1°C
So, by substituting we have:
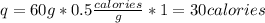
So, 60 grams of ice will require 30 calories to raise the temperature 1°C
Have a nice day!