Answer:

Step-by-step explanation:
First of all, we need to calculate the energy of one photon with wavelength 600 nm. The energy of each photon is given by

where
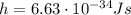 is the Planck's constant
is the Planck's constant
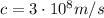 is the speed of light
is the speed of light
 is the wavelength of the light
is the wavelength of the light
Substituting numbers into the formula, we find

Now to find the number of photons, we simply divide the total energy (250 J) by the energy of one photon:
