Answer : The total pressure in the cylinder is 7.5 atm.
Solution :
According to the Dalton's law, the total pressure of the gas is equal to the sum of the partial pressure of the mixture of gasses.
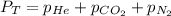
where,
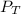 = total partial pressure in the cylinder = ?
= total partial pressure in the cylinder = ?
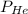 = partial pressure of helium = 4.21 atm
= partial pressure of helium = 4.21 atm
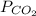 = partial pressure of carbon dioxide = 0.6 atm
= partial pressure of carbon dioxide = 0.6 atm
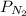 = partial pressure of nitrogen = 2.7 atm
= partial pressure of nitrogen = 2.7 atm
Now put all the given values is expression, we get:
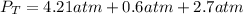
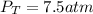
Therefore, the total pressure in the cylinder is 7.5 atm.