Answer:
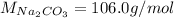
Step-by-step explanation:
Hello,
In this case, we should remember that molar mass is obtained via adding the product between the atomic mass and the subscript regarding with each element forming the compound. Thus, for sodium carbonate we have:
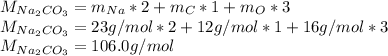
Best regards.