Answer: Option (C) is the correct answer.
Step-by-step explanation:
A precipitation reaction is a reaction in which two aqueous solution chemically react with each other resulting in the formation of an insoluble solid.
This insoluble solid is known as precipitate.
For example,
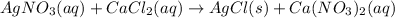
In this reaction, AgCl is the precipitate that is formed.
Therefore, we can conclude that in solid state the precipitate exist in a precipitation reaction.