Molar mass of the gas:
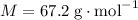 .
.
Step-by-step explanation
How many moles of gas molecules in that 300 ml of gas sample at STP?
The gas is under Standard Temperature and Pressure, STP. Each mole of an ideal gas will occupy a volume of around 22.4 L or 22,400 ml.
By Arrhenius's Law, the volume of an ideal gas is proportional to the number of gas particles in that gas.
Assuming that the gas in question here is ideal.
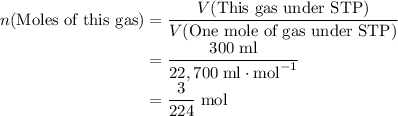 .
.
What's the molar mass of this gas?
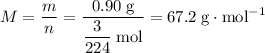 .
.