Answer:
A. 1.73 x 10^5 J
Step-by-step explanation:
The internal energy of a gas is related to the average speed of the molecules of the gas. In fact:
- The internal energy, U, is directly proportional to the temperature of the gas, T, according to
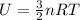
- The temperature of the gas, T, is proportional to the square of the average speed of the molecules, v, according to
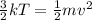
where both terms represent the average kinetic energy of the molecules.
- Therefore, we can conclude that the higher the internal energy, the faster the average speed of the molecules: among the choices given, the sample with highest internal energy is sample A.