Answer:
The mass of CO2 produced by one liter of fuel is 2,200 gr/lt
Explanation:
Proportions
To find the cross-relation between the mass of CO3 produced and the liters of fuel used we must find the unit rate of both relationships given.
We know:
The car uses 5 liters of fuel per 100 Km. The unit rate is 5/100 lt/km
The car produced 110 grams of CO2 for each kilometer traveled. The unit rate is 110 gr/Km
Since the kilometers is the common unit, we find the unit rate of CO2/fuel:
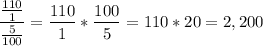
The mass of CO2 produced by one liter of fuel is 2,200 gr/lt