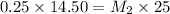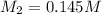Answer: The concentration of the hydrochloric acid is 0.145 M.
Step-by-step explanation:
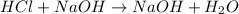
According to given balanced equation, 1mole of HCL require 1 mole of NaOH.
Using Molarity equation:

 = Molarity of base= 0.25 M
= Molarity of base= 0.25 M
 = volume of base= 14.50 ml
= volume of base= 14.50 ml
 = Molarity of acid = ? M
= Molarity of acid = ? M
 = volume of acid = 25 ml
= volume of acid = 25 ml