Step-by-step explanation:
B. 1.00 x 10^-2 M HCI
The question is asking us to find the molarity/concentration of the acid (HCl).
We have been provided with;
- 20.0 mL of 5.0 x 10^-³ M NaOH
- 10.0 mL of HCl
We know that molarity (M) of a solution is contained in 1 L or 1000 mL or 1000 cm³.
This means that;
5.0 x 10^-³ moles is contained in 1000 mL.
is contained in 1000 mL.X mol is contained in 20.0 mL
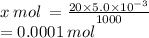
= 0.0001 moles
These 0.0001 moles is contained in 10 mL of HCl.
To find the molarity of the acid;
0.0001 moles is contained in 10 mL
x mol is contained in 1000 mL
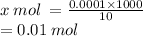
= 0.01 M
Therefore the Concentration of the acid (HCl) is 0.01 M or 1.0 x 10^-2 M