Answer: 44.04 g/mol
Step-by-step explanation:
Ideal gas law in terms of density:
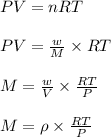
where,
P = pressure of the gas = 0.789 atm
V = volume of the gas
T = temperature of the gas =
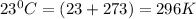
n = number of moles of the gas
R = gas constant = 0.0821 Latm/moleK
M = molar mass of gas
w = mass of gas
 = density =1.43 g/ml
= density =1.43 g/ml
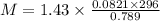
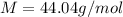
Thus molar mass of the gas is 44.04 g/mol