Answer: The chemical compound formed by the combination of given ions is
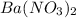
Step-by-step explanation:
Barium is the 56th element of periodic table having electronic configuration of
![[Xe]6s^2](https://img.qammunity.org/2020/formulas/chemistry/middle-school/14psnspetimewzf4si7rpt06do00n9r2em.png) .
.
To form
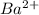 ion, this element will loose 2 electrons.
ion, this element will loose 2 electrons.
Nitrate ion is a polyatomic ion having chemical formula of
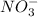
By criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
So, the chemical compound formed by the combination of given ions is
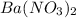
The formed compound is an ionic compound.