5.3 L.
Step-by-step explanation
By Charles's Law,
- the volume of an ideal gas is proportional to its absolute temperature when its pressure is held constant.
In other words,
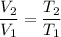 ,
,
where
 is the volume of the gas, and
is the volume of the gas, and
 is the absolute temperature of the gas in degrees Kelvins.
is the absolute temperature of the gas in degrees Kelvins.
As a noble gas, helium is quite "ideal" with rather weak attractions between its particles unless in direct contact.
Convert the temperatures:
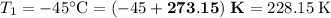 ;
;
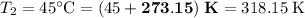 .
.
Apply Charles's Law,
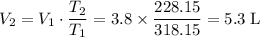 .
.