Answer: The ratio of atoms in calcium bicarbonate ; Ca : H : C : O = 1:2:2:6.
The ratio of atoms in lithium sulfide; Li : S = 2 : 1
Step-by-step explanation:
In calcium bicarbonate:
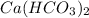
In a molecular formula of calcium carbonate there are:
Number of Calcium atoms = 1
Number of Hydrogen atom = 1 × 2 = 2
Number of Carbon atoms = 1 × 2 = 2
Number of Oxygen atoms = 3 × 2 = 6
So, Ca : H : C : O = 1 : 2 : 2 : 6
In lithium sulfide :
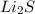
In a molecular formula of lithium sulfide there are:
Number of Lithium atoms = 1 × 2 = 2
Number of Sulfur atoms = 1
So, the Li : S = 2 : 1