Answer : The correct option is, 34 L
Explanation : Given,
Moles of oxygen gas = 1.5 mole
As we know that,
At standard temperature and pressure (STP), 1 mole of gas contains 22.4 liters volume of gas
As, 1 mole of oxygen gas contains 22.4 liters volume of oxygen gas
So, 1.5 mole of oxygen gas contains
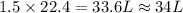 volume of oxygen gas
volume of oxygen gas
Therefore, the volume of oxygen gas at standard temperature and pressure (STP) is, 34 L