Step-by-step explanation:
When the student mixed the solution sodium carbonate with solution of copper(II) sulfate ; Copper Hydroxocarbonate , sodium sulfate and carbon dioxide gas was obtained as a products.
The balanced chemical reaction
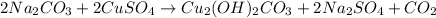
Where:
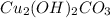 = Copper(II) Hydroxocarbonate
= Copper(II) Hydroxocarbonate
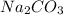 = Sodium carbonate
= Sodium carbonate
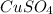 = Copper(II) sulfate
= Copper(II) sulfate
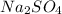 = Sodium sulfate
= Sodium sulfate
 = Carbon-dioxide
= Carbon-dioxide