Answer: 1. Silicon dioxide
2.
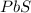
3. Magnesium chloride
Step-by-step explanation: For naming binary covalent compounds like
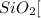 The name of the non-metal present on the left in the periodic table is written followed by the name the other non-metal by its elemental name ending with -ide. Use the prefix mono-, di-, tri-.... to indicate the number of the other non metal in the molecule.
The name of the non-metal present on the left in the periodic table is written followed by the name the other non-metal by its elemental name ending with -ide. Use the prefix mono-, di-, tri-.... to indicate the number of the other non metal in the molecule.
For binary ionic compounds, like lead sulfide and
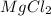 , The cation is named first, and then the anion is named. Cations are named by their elemental name and anions are named by their elemental name and ends with "-ide" without any prefix or suffix.
, The cation is named first, and then the anion is named. Cations are named by their elemental name and anions are named by their elemental name and ends with "-ide" without any prefix or suffix.