The right answer is
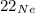 , Neon 22.
, Neon 22.
The second rare gas in the periodic table, neon, has an atomic number of 10, it gave its name to neon tubes and to a variety of fluorescent tubes.
Neon has three stable isotopes, 20Ne (the most abundant), 21Ne, and 22Ne, and a standard atomic mass of 20,1797 u.