Answer : The element is, Arsenic (As)
Solution : Given,
Mass of a pure element = 4.17 g
Number of atoms =
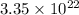
First we have to calculate the moles of a pure element.
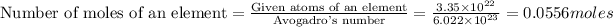
Now we have to calculate the molar mass of an element.
Formula used :
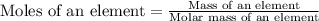
Now put all the given values in this formula, we get
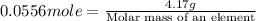
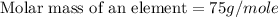
Thus, the arsenic is the element which has molar mass 75 g/mole.
Hence, the element is, Arsenic (As)