Given that the molality of the Iron(III) chloride solution = 1.541 mol /kg water
1.541 mol
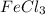 is present per L solution
is present per L solution
Calculating mass of
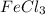 from 1.541 mol:
from 1.541 mol:
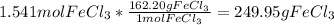
Mass of solvent = 1 kg
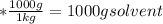
Total mass of the solution = 1000 g + 249.95 g = 1249.95 g solution
Density of the solution =
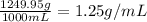
Molarity of the solution = 1.457 mol/L solution
Calculating mass of
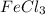 from 1.457 mol:
from 1.457 mol:
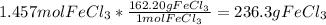
Volume of the solution = 1 L *
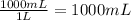
Mass of solution =
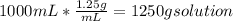
Mass percentage of
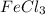 in the solution =
in the solution =
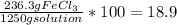
Therefore density of the solution is 1.25 g/mL
Mass percent of the solution is 18.9 %