Density is the amount of mass per unit volume. The volume of water here is fixed at 100 mL, so its density is determined entirely by its mass:
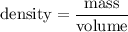
a. By definition, density is directly proportional to mass, meaning the higher the mass, the higher the density. As the table suggests, higher temperatures lead to lower mass, which in turn means lower density.
b. According to the table, the highest density occurs at a temperature of 4C.
c. I assume "cold" means the water is initially at a temperature slightly above its freezing point (0C). As the table suggests, the mass of water decreases as temperature rises. Prior to reaching its boiling point (100C), you can expect the water level to rise to the point of overflowing from the beaker.