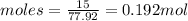We are given with the mass of Arsine (
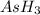
The mass of arsine is 15g
there is a relation between moles, mass and molar mass of any compound which is
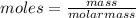
The molar mass of Arsine = atomic mass of As + 3X atomic mass of H
the molar mass of Arsine = 74.92 + 3X 1 = 77.92 g/mol
Let us calculate the moles as