Generally we have to kinds of conductors
a) metallic conductors: which conduct electricity due to free movement of electrons
b) electrolytic conductors: which conduct electricity due to movement of ions.
HCN is an electrolyte and is non conducting in pure state as it has no free ions or electrons
When HCN is dissolved in water it dissociates as
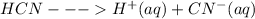
These ions obtained due to dissociation of HCN makes it conducting in nature.