Answer:
B.
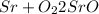
Step-by-step explanation:
Balanced chemical equation: when number of atoms of given element in the reactant is equal to number of atoms of the element in the product.Then the chemical equation is balanced.
We are given that some chemical equation.
We have to find chemical unbalanced equation.
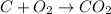
On left side
Number of carbon=1
Number of oxygen=2
On right side
Number of carbon=1
Number of oxygen=2
It is balanced chemical equation because number of carbons and oxygen are equal on both sides.
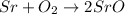
On left side
Number of atom of Sr=1
Number of oxygen=2
On right side
Number of atom of Sr=2
Number of oxygen=2
It is unbalanced chemical equation because number of Sr is not equal on both sides.
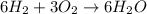
Number of oxygen on LHS = Number of oxygen on RHS=6
Number of hydrogen on LHS =Number of Hydrogen on RHS=12
Number of oxygen and Hydrogen are equal on both sides.Therefore, the chemical equation is balanced.
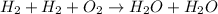
Number of oxygen on LHS=Number of oxygen on RHS=2
Number of Hydrogen on LHS=Number of Hydrogen on RHS=4
It is balanced chemical equation because number of hydrogen and oxygen on both sides are equal.