Answer:The solubility product expression for
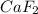 .
.
![K_(sp)=[Ca^(2+)]* [F^-]^2](https://img.qammunity.org/2020/formulas/chemistry/high-school/f824n3wzrblyolcl48s9eym90rimkiydjm.png)
Step-by-step explanation:
Solubility product is defined as product of concentration of dissociated ions raised to the power equal to respective stoichiometric coefficient.
The solubility product of
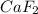
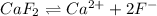
![K_(sp)=[Ca^(2+)]* [F^-]^2](https://img.qammunity.org/2020/formulas/chemistry/high-school/f824n3wzrblyolcl48s9eym90rimkiydjm.png)
The solubility product expression for
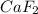 .
.
![K_(sp)=[Ca^(2+)]* [F^-]^2](https://img.qammunity.org/2020/formulas/chemistry/high-school/f824n3wzrblyolcl48s9eym90rimkiydjm.png)