Answer: There are
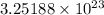 ammonium ions and
ammonium ions and
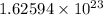 sulfate ions in the given amount of ammonium sulfate.
sulfate ions in the given amount of ammonium sulfate.
Step-by-step explanation:
We are given 0.270 moles of ammonium sulfate and we need to find the number of ammonium and sulfate ions in it. The equation for the ionization of ammonium sulfate is given by the reaction:
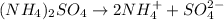
By Stoichiometry,
1 mole of ammonium ions dissociates into 2 moles of ammonium ions and 1 mole of sulfate ion.
According to the mole concept:
1 mole of an ionic compound contains
 number of ions.
number of ions.
Hence, 0.270 mole of ammonium sulfate will contain
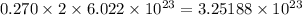 number of ammonium ions and
number of ammonium ions and
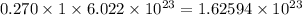 number of sulfate ions.
number of sulfate ions.
Hence, there are
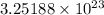 ammonium ions and
ammonium ions and
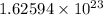 sulfate ions in the given amount of ammonium sulfate.
sulfate ions in the given amount of ammonium sulfate.