Answer: Aluminium has 3, Sulfur has 6, Sodium has 1, Silicon has 4, Magnesium has 2 and Phosphorous has 5 valence electrons.
Step-by-step explanation:
Valence electrons are defined as the electrons which are present in the outermost shell of an element. This can be determined very well from the electronic configurations of the elements.
The given elements are the elements which belong to the same period which is Period 3.
Electronic configuration of the given elements:
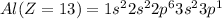
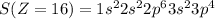
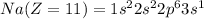
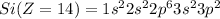
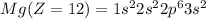
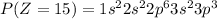
Hence, from the electronic configurations, Aluminium has 3, Sulfur has 6, Sodium has 1, Silicon has 4, Magnesium has 2 and Phosphorous has 5 valence electrons.