Answer: The percent composition of tungsten in tungsten carbide is 94%.
Step-by-step explanation:
Percent composition of an element in a compound is defined as the mass of the element present in 1 mole of a compound.
Mass of 1 mole of WC = 196 g/mol
Mass of tungsten = 184 g/mol
The percent composition of tungsten in WC =
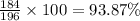
Rounding this off to the nearest whole percent, we get:
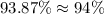
Hence, the percent composition of tungsten in tungsten carbide is 94%.