The electrons are transferred when the reaction is redox reaction. It means that there must be reduction and oxidation.
The given equation is
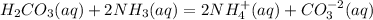
The oxidation number of each element remains the same before and after the reaction.
H = +1
C: +4
O: -2
N: -3
Thus this is simple acid base reaction and not redox
There is transfer of proton from bicarbonate to ammonia
this is not a redox reaction