Answer:- 0.138 M
Solution:- The buffer pH is calculated using Handerson equation:
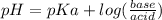
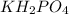 acts as a weak acid and
acts as a weak acid and
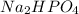 as a base which is pretty conjugate base of the weak acid we have.
as a base which is pretty conjugate base of the weak acid we have.
The acid hase two protons(hydrogen) where as the base has only one proton. So, we could write the equation as:
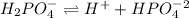
Phosphoric acid gives protons in three steps. So, the above equation is the second step as the acid has only two protons and the base has one proton.
So, we will use the second pKa value. The acid concentration is given as 0.10 M and we are asked to calculate the concentration of the base to make a buffer of exactly pH 7.00.
Let's plug in the values in the equation:
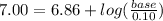
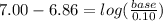
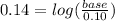
Taking antilog:
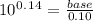
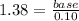
On cross multiply:
[base] = 1.38(0.10)
[base] = 0.138
So, the concentration of the base that is
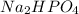 required to make the buffer is 0.138M.
required to make the buffer is 0.138M.