Answer:- D. Double- replacement reaction
Explanations:- In general, the decomposition reactions looks like:
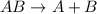
The given reaction looks different than this and so it is not decomposition reaction.
A single-replacement reaction looks like:
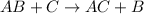
Our reaction looks different from this so single-replacement is also not correct.
Synthesis reaction looks exactly opposite to decomposition reaction.
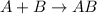
The given reaction is different than this and so it is not synthesis reaction also.
A double-replacement reaction looks like:
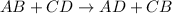
In this reaction, the ions are exchanged as could be seen in the general reaction. On reaction side,A is with B and C is with D. On product side, A is with D and C is with B.
The same is happening in the given reaction. Nitrate ion is with lead metal on reactant side and chlorine is with hydrogen, On product side, Chlorine is with lead metal and nitrate ion is with hydrogen. So, ion exchange has taken place here and so this is a double-replacement reaction and choice D is correct.