Here, we have to get the number of atoms present in the 100 plane of the FCC crystal lattice.
There will be 2 atoms in 100 plane of FCC crystal lattice.
In the face centered crystal (FCC) lattice there are atoms at each corner of the cube and each are shared by 4 another atoms. And an atom is present at the face of the crystal.
For the 100 plane of the Miller indices the intercepts are a, ∞, ∞ or 2a, ∞, ∞.
Thus, for the 4 atoms of the corner at the cube shared by 4 other atoms will contribute, 4 ×
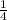 = 1 and the un-shared atoms at the face will contribute another 1, which make the total atom 1 + 1 = 2.
= 1 and the un-shared atoms at the face will contribute another 1, which make the total atom 1 + 1 = 2.