Answer: The correct answer is the soda cap is denser than rubbing alcohol but not denser than the vinegar and glycerin.
Step-by-step explanation:
Density is defined as the ratio of mass of object to its volume. The mathematical expression of density is given as:
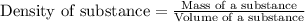
A substance having more density will sink at the bottom and the substance having less density will float at the top.
In a beaker, the bottom layer is of Glycerin, then vinegar. Soda cap is in between the layers of vinegar and rubbing alcohol. This means that, vinegar has more density than soda cap. The top most layer is of rubbing alcohol.
Thus, the order of density is as follows:
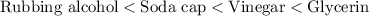
Hence, the correct answer is the soda cap is denser than rubbing alcohol but not denser than the vinegar and glycerin.