Answer: The final volume will be 14.85 L.
Step-by-step explanation:
To calculate the final volume when temperature increases, we use Charles' Law.
This law states that volume is directly proportional to the temperature of the gas if number of moles and pressure remains constant.
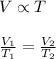
where,
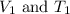 = Initial volume and temperature
= Initial volume and temperature
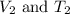 = Final volume and temperature
= Final volume and temperature
We are given:
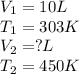
Putting values in above equation, we get:
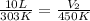
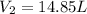
Hence, the final volume of the gas is 14.85L