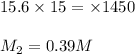Answer:
For 1: 19.52mL of the stock solution must be taken.
For 2: The concentration of the final solution will be 0.39M
Step-by-step explanation:
To calculate the volume or molarity of the solution, we use the equation:

where,
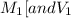 are the molarity and volume of one solution
are the molarity and volume of one solution
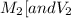 are the molarity and volume of another solution
are the molarity and volume of another solution
 = Molarity of the stock solution = 15.6M
= Molarity of the stock solution = 15.6M
 = Volume of the stock solution = ? mL
= Volume of the stock solution = ? mL
 = Molarity of the diluted solution = 0.210M
= Molarity of the diluted solution = 0.210M
 = Volume of the diluted solution = 1450 mL
= Volume of the diluted solution = 1450 mL
Putting values in above equation, we get:
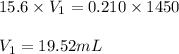
 = Molarity of the stock solution = 15.6M
= Molarity of the stock solution = 15.6M
 = Volume of the stock solution = 15 mL
= Volume of the stock solution = 15 mL
 = Molarity of the diluted solution = ? M
= Molarity of the diluted solution = ? M
 = Volume of the diluted solution = 0.600L = 600mL (Conversion factor: 1L = 1000mL)
= Volume of the diluted solution = 0.600L = 600mL (Conversion factor: 1L = 1000mL)
Putting values in above equation, we get: