Answer:50.4 moles of atoms are present in the sample.
Step-by-step explanation:
Moles of carbon atom in propane = 14.1 moles of atom
Number carbon atoms
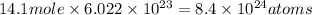
In one molecule of propane there are 3 carbon atoms and 8 hydrogen atoms
For 1 carbon atom there are =
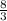 hydrogen atoms
hydrogen atoms
Number of hydrogen atom for
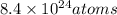 of carbon :
of carbon :
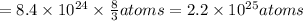
Total number of atoms =
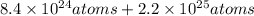
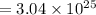 atoms
atoms
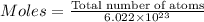
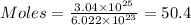
50.4 moles of atoms are present in the sample.