Answer:- 13.6 L
Solution:- Volume of hydrogen gas at 58.7 Kpa is given as 23.5 L. It asks to calculate the volume of hydrogen gas at STP that is standard temperature and pressure. Since the problem does not talk about the original temperature so we would assume the constant temperature. So, it is Boyle's law.
Standard pressure is 1 atm that is 101.325 Kpa.
Boyle's law equation is:
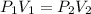
From given information:-
 = 58.7 Kpa
= 58.7 Kpa
 = 23.5 L
= 23.5 L
 = 101.325 Kpa
= 101.325 Kpa
 = ?
= ?
Let's plug in the values and solve it for final volume.
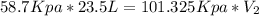
On rearranging the equation for

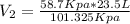
 = 13.6 L
= 13.6 L
So, the volume of hydrogen gas at STP for the given information is 13.6 L.