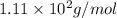Answer: The correct option is
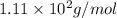
Step-by-step explanation:
Atomic mass of calcium ,Ca = 40.07 g/mol
Atomic mass of chlorine ,Cl = 35.5 g/mol
Molar mass of
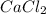 :
:
= (Atomic mass of Ca+2 × (Atomic mass of Cl))
= (40.07 g/mol + (2 × 35.5))=111.07 g/mol
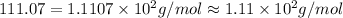
Hence, the correct option is